Day 1 :
Keynote Forum
Roger M Leblanc
University of Miami, USA
Keynote: Development and bio-applications of nontoxic C-Dots
Time : 10:00-10:30

Biography:
Roger M. Leblanc received a B.Sc. degree in Chemistry from Université Laval in 1964, followed by a PhD in Physical Chemistry in 1968. Then, he obtained a postdoc position at the Royal Institution of Great Britain for two years before moving to the University of Québec, Trois-Rivières, Canada, where he spent 20+ years of studying photobiophysics. He moved his research to the University of Miami in 1994. Dr. Leblanc is Professor and Chair of Chemistry Department at University of Miami. And his research interests are centered on biophotophysics, spectroscopy and surface chemistry and he has published more than 500 research articles related to these topics and has guided more than 100 Ph.D. and M.Sc.
Abstract:
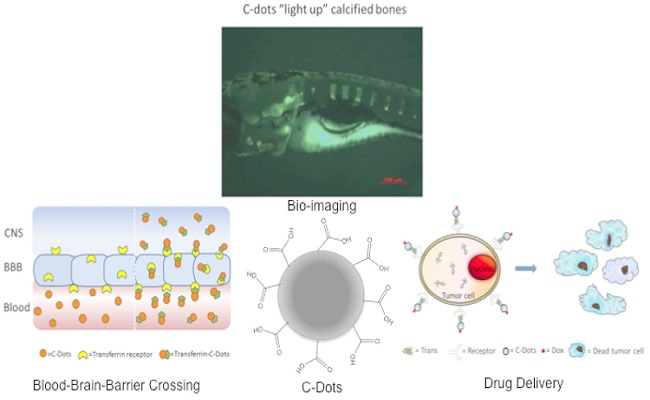
Carbon dots (C-Dots) have recently attracted enormous attention due to their unique properties. In this talk, the synthesis, characterization and bio-applications of a new type of nontoxic, water-soluble C-Dots will be presented. A major medical challenge one faces to treat central nervous system (CNS) related diseases is to cross the tight junctions between endothelial cells, which are known as blood–brain barrier (BBB). Recently, our in vivo experimental observations suggested that the transferrin conjugated C-Dots could enter the CNS of Zebrafish while C-Dots alone could not. Thanks to the abundant presence of carboxylic acids on the surface, C-Dots are easily conjugated with transferrin and anticancer drug doxorubicin. The system was then applied as a drug delivery system for the delivery of doxorubicin into cancerous cells. Our in vitro study showed greater uptake of the conjugates compared to free doxorubicin, the conjugates at 10 nM was significantly more cytotoxic than doxorubicin alone, reducing viability by 14~45 %, across multiple pediatric brain tumor cell lines. Accidents, disease and aging compromise the structural and physiological functions of bones, and in vivo bone imaging test is critical to identify, detect and diagnose bone related development and dysfunctions. Here we show that C-Dots with low quantum yield (“dark”) bind to calcified bone structures of live Zebrafish larvae with high affinity and selectivity. Binding resulted in a strong enhancement of luminescence that was not observed in other tissues, including non-calcified endochondral elements. Retention of C-dots by bones was very stable, long lasting, and with no detectable toxicity. These observations support a novel and revolutionary use of C-Dots as highly specific bioagents for bone imaging and diagnosis, and as a potential bone-specific drug delivery carrier.
Keynote Forum
Miroslav Ryska
Quinta-Analytica, Czech Republic
Keynote: Can matrix effect in LC/MS or LC/MS/MS assay be avoided or fully compensated?
Time : 11:20-11:50

Biography:
Miroslav Ryska holds an Undergraduate Degree from Charles University, along with an MS in Physical Chemistry from Moscow State University and a PhD from the Institute of Macromolecular Chemistry of Czechoslovak Academy of Sciences. From 1961 to 1978, he worked at the Institute of Macromolecular Chemistry of the Czechoslovak Academy of Sciences. From 1978-1997, he worked as a Researcher in the Research Institute for Pharmacy and Biochemistry in Prague. He has written more than 100 publications mainly on the topic of mass spectrometry, trace analyses, analyses of drugs, metabolites and quantitative analysis. Currently he is in the position of Scientific Advisor.
Abstract:
The source of the “Matrix effect” as a consequence of analyte ions suppression or ions enhancement must be sought in the presence of unknown impurities from matrix. They are participating in the complex ionization process in parallel or competing ion-molecular reactions. Not only impurities from extracts but impurities adsorbed in the ion source and/or in the analytical system may play an important role. These adsorbed substances cannot be fully removed from the system by any cleaning procedure. To fully compensate for the negative impact of the “Matrix effect“, use of isotopically labeled internal standards (isotope dilution technique) are proved to be the only effective technique. This applies especially to LC/MS/MS determination of drugs and their metabolites in complex extracts of biological matrices. The isotope dilution technique is successful regardless of the method of purification, the ionization technique (APCI or ESI) and the type of the equipment used. The isotope dilution technique proved to be 100% effective for the compensation of matrix effect influences in 132 analytical methods developed and validated. The strict requirements of EMA guidelines to investigate different plasma sources for the assessment of the matrix effect in the analytical method validation are discussed
- Pharmaceutical Reasearch, Development and Technology | Advances and Applications in HPLC Techniques | Analytical Chemistry | Quality Control, Quality Assurance and Regulatory Filings | Pharmaceutical Nanotechnology | Methods of Chromatography
Location: Fleming’s 7+8

Chair
Salah M Blaih
Kent State University, USA

Co-Chair
M Sharaf El-Din
University of Mansoura, Egypt
Session Introduction
Salah M Blaih
Kent State University, USA
Title: Personalized cancer therapeutics: Updates on drug developments and characterization of biosimilars

Biography:
Salah M Blaih has been involved in teaching, research and authoring in pharmacy and chemistry for more than 25 years. He was elected to the USP’s Council of Expert’s Committee on Gastrointestinal, Renal, and Endocrine and is a world-wide invited speaker on quality of medicines/compendia standards, pharmacogenomics, and medication safety. He is a Fellow of The Royal Society of Chemistry, a two-time president of The American Chemical Society-Penn Ohio Section, and a board-certified pharmacist with biographical entries in Who’s Who in the World and Who’s Who in America.
Abstract:
Cancer biomarkers are relevant for identifying patients who are likely to benefit from a given treatment (right drug for the right patient). This approach is utilized in cancer drug development as well as in measuring patient’s response to therapy. Targeted therapies are designed to interfere with molecular targets with a goal of more precision and fewer side effects. These molecular pathways are broadly classified as either 1) monoclonal antibodies that target transmembrane receptors or extracellular growth factors or 2) small molecules that penetrate cell membrane and block and interfere with the enzymatic activity of target proteins. Emerging cancer therapeutics, which act on specific molecular targets, their mutations, and their role in tumor progression are presented: 1) PI3K, CDK, and PARP inhibitors and 2) PD-1 (programmed death receptor binding). Some aspects of development, characterization, and regulation of biosimilars as implemented by the FDA and EMEA are also presented.
M Sharaf El-Din
University of Mansoura, Egypt
Title: Spectrophotometric methods for simultaneous determination of rivaroxaban and clopidogrel in their binary mixture

Biography:
Professor Dr.Mohie Sharaf El Din has completed his PhD at the age of 32 years from Bonn -University – Germany 1982 and post-doctoral studies from Graz Institute of pharmaceutical chemistry and biochemistry 1986 - Austria. He was the Dean of Faculty of Pharmacy – Heliopolis University – Cairo-Egypt 2015, Head of Analytical chemistry Department, Faculty of Pharmacy, and Mansoura University – Mansoura – Egypt 1997 -2004. He has published more than 100 papers in reputed journals and has been serving as an editorial board member of repute.( as an editorial board member of Mansoura Journal of pharmaceutical Science , Faculty of Pharmacy - Mansoura – Egypt, as the chair/co-chair for the session of many conferences and workshops )
Abstract:
Three rapid, accurate and very simple derivative spectrophotometric methods for RIV and CLP assay in their binary mixture and tablet dosage forms were developed. Method (I) is first derivative spectrophotometric method, derivative amplitudes were measured at the zero crossing wavelength of 289 and 249.5 nm for determination of RIV and CLP, respectively. The calibration curve is rectilinear over the range 2.0-20.0 µg/ml for RIV and 5.0-60.0 µg/ml for CLP with LOD of 0.211 and 0.361 μg mL-1 and LOQ of 0.641 and 1.095 μg mL-1 for RIV and CLP, respectively. Method (II) is ratio derivative spectrophotometric method. The ratio spectra of each drug were derived by dividing its spectra on a constant concentration of the other drug as a divisor. Derivative amplitudes were measured at 256 nm for RIV and at 214.5 nm for CLP over the same concentration range as the first method with LOD of 0.137 and 0.485 μg mL-1 and LOQ of 0.417and 1.471 μg mL-1 for RIV and CLP, respectively. Method (III) is absorbance ratio method, absorbance of both drugs were recorded at two wavelengths λ1 (232) iso-absorptive point and λ2 (249) λmax of RIV. The final concentrations were obtained by applying the Q equations. The method was linear over the same concentration range as the first method with LOD of 0.272 and 0.485 μg mL-1 and LOQ of 0.826 and 1.471 μg mL-1 for RIV and CLP, respectively. The proposed methods were validated as per International Conference of Harmonization guidelines. The proposed methods were successfully applied to both drugs analysis in their laboratory prepared co-formulated tablet. Statistical comparison of the results with those of the reference method illustrate good agreement and confirm that there were no significant difference in the accuracy and precision between the proposed and reference one respectively.
Thomas D Benen
Microtrac GmbH, Germany
Title: Integrating 180° DLS into on-line pharmaceutical processes and high-throughput robotics

Biography:
Thomas D Benen has completed his PhD at the University of Hamburg and Postoctoral Research at the University of Regensburg, investigating the formation of viral particles. He has worked for some of the major players of the particle characteriztation industry like Malvern Instruments and NanoSight Ltd. At Microtrac GmbH, he is responsible for the business in the D-A-CH region. He is delegate of the ISO group TC24 committed on the develeopment of international standards for particle measurement.
Abstract:
Dynamic Light Scattering (DLS) is a prevalent tool for determining particle size distributions in fine particulate material suspensions, micro-emulsions and nano-scale matter like proteins and drug delivery particles. Usually optical arrangements in lower angles are used that demand heavy dilution of samples. Backscattering, however, allows for considerably higher concentrations and is the right choice for concentrated samples which incur in processes. Furthermore, the 180° backscattering is especially suitable for direct in-line use in reactors, because the handling of the measurement probe head with a diameter of 8 mm is just as easy as a pH head. A probe cap effectively shields the Brownian motion from the process fluidics. At higher concentrations, where particle-particle interactions are present, on-line systems with automated dilution are in use. The external control of our DLS measurement software moreover allows for using robots in High-Throughput Screening (HTS) and fully automated formulation stations. Those work stations, equipped with liquid handling and parallel reactors, perform programmed formulation steps like mixing, stirring, heating and shaking. Apart from the aforementioned particle size distribution, rheological attributes and spectroscopic measurements are also included. Thus DLS has finally arrived as an automatized tool for pharmaceutical processes and HTS.
Roger M Leblanc
University of Miami, USA
Title: Analysis of microbiomolecule-nanoparticle drug delivery system using circular dichroism spectroscopy

Biography:
Roger M. Leblanc received a B.Sc. degree in Chemistry from Université Laval in 1964, followed by a Ph.D. in Physical Chemistry in 1968. Then, he obtained a postdoc position at the Royal Institution of Great Britain for two years before moving to the University of Québec, Trois-Rivières, Canada, where he spent 20+ years of studying photobiophysics. He moved his research to the University of Miami in 1994. Dr. Leblanc is Professor and Chair of Chemistry Department at University of Miami. And his research interests are centered on biophotophysics, spectroscopy and surface chemistry and he has published more than 500 research articles related to these topics and has guided more than 100 Ph.D. and M.Sc.
Abstract:
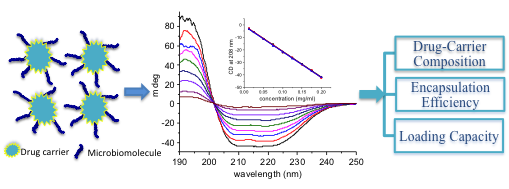
Microbiomolecules such as peptides, proteins and nucleic acids (i.e., DNA and RNA) have become very promising drug candidates in recent decades due to their unique properties and interactions with biological systems. Compared to the traditional small organic molecules (i.e., less than 500 g/mol), these candidates are highly selective due to their rich structure features and unique interaction with their target. However, due to their high enzymatic susceptibility, low membrane permeability and poor bioavailability, applications of these drugs have been significantly limited. Targeted drug delivery by drug delivery systems (DDS) has been the main strategy to overcome the problems (i.e., high enzymatic susceptibility and poor bioavailability) associated with these microbiomolecules drug candidates. In this regard, considerable efforts have been made to design and develop drug delivery systems that could transport these microbiomolecules to site of interest. One important aspect to consider when designing a DDS is the drug-to-carrier ratio since the use of high quantity of carrier could potentially cause a series of problems, such as carrier-related toxicity and the possibility of immune reactions against the carrier in the patient body. However, the ability to determine the composition of a DDS after loading a drug to the carrier is significantly limited by current analytical methods. In this talk, a simple yet convenient method based on circular dichroism (CD) spectroscopy to determine the compositions of the various protein-carrier conjugates will be introduced. Specifically, five important proteins, α1-antitrypsin, hemoglobin human, human serum albumin, human transferrin and r-globulin were chemically conjugated to two model drug carriers, namely carbon dots and polymer O-(2-carboxyethyl) polyethylene glycol; and their compositions were determined with CD spectroscopy. It will also be demonstrated that the composition of nucleic acid conjugates could also be determined using the same methodology.
Nahed El-Najjar
University Hospital Regensburg, Germany
Title: Liquid chromatography-tandem mass spectrometry increases the clinical outcome from the antimicrobial therapy used in intensive care units

Biography:
Abstract:
In the last years, liquid chromatography-tandem mass spectrometry (LC-MS/MS) has become the method of choice for therapeutic drug monitoring (TDM) of drugs used in critically ill patients. This is largely due to the high accuracy and fast delivery of the results obtained with LC-MS/MS which enables quick decision in dose adjustment increasing thus the clinical outcomes of treatments used in Intensive Care Units (ICU) patients. This is particularly of interest when it comes to the antibiotic therapy which is increasingly acknowledged to be frequently unsatisfactory. Inadequacy of the antibiotic therapy is due to the wide range of pathophysiological changes observed in critically ill patients, which impact the proper pharmacokinetic/pharmacodynamic profile of the antibiotics used. Even though the observed poor clinical outcome is mostly related to poor antibiotics exposure, which also increases the risk of acquiring bacterial resistance, dysfunction in kidney and liver, which are the major routes for drug elimination, result in an increased risk of the prompt onset of toxic drug concentration. Therefore, establishing reliable methods for TDM is of utmost importance. When it comes to be applied in routine analysis, analytical methods used for TDM should encompass the simultaneous analysis of different antibiotics widely used in ICU. Challenges encountered during method development as well as the successful application of LC-MS/MS methods for TDM of antibiotics in critically ill patients will be presented and discussed.
Yuan-Po Lee
Chia-Nan University of Pharmacy and Science, Taiwan
Title: The evaluation of stir-bar sorptive extraction and dispersive liquid-liquid microextraction methods for the determination of 20 pharmaceuticals in environmental waste water
Biography:
The Authors are all teaching in Chia-Nan University of Pharmacy and Science and worked on the analytical methods development and validation for more than 20 years. The scope for the analytical methods have aplplied to pharmaceutical, cosmetic and environmental samples and offer industrial services
Abstract:
Stir bar sorptive extraction, SBSE and Dispersive liquid-liquid microextraction, DLLME have attracted much interest due to their simplicity, rapidity of operation by allowing the direct extraction of solutes from sample or low consumption of solvents and reagents during sample pretreament. In this research, SBSE and DLLME were used for the sample pretreament of environmental water samples and followed by gas chromatography/mass spectroscopy analysis for the validation of determining 20 pharmaceuticals in environmental water samples. Fluoxetine, chlorpheniramine, sertraline, methamphetamine, diphenhydramine, amitriptyline, lidocaine, venlafaxine, citalopram, chlorpromazine, verapamil, propoxyphene, promethazine, diazepam, meperidine, methadone, doxylamine, mirtazapine, dextomethorphan and codeine were selected as the target compounds. The results show that most substances can be effectively extracted by SBSE at pH 9.2 except methamphetamine, lidocaine and codeine and by DLLME using toluene as extraction solvent except methamphetamine, propoxyphene, codeine and diazepam. The applicability of the sample pretreatment methods strongly depended on the characteristics of target compounds. Codeine shows very poor recoveries which means it is not suitable to determine its content under selected analytical conditions. It is recommended to use SBSE methods for sample pretreatment because it can achieve lower detection limit, repeated use of stir bar and no need to use extraction solvent. For real environmental water samples, SBSE was used to determine the presence of target compounds and evaluate the matrix effect of the real samples. The results show that only lidocaine was detected in one of the hospital waste samples and methamphetamine, lidocaine and codeine all showed poor recoveries as in standard solution.
Somjing Roongjang
Chiang Mai University, Thailand
Title: The stability of cefoperazone/sulbactam (sulperazone) in PD solutions

Biography:
Somjing Roongjang has completed his Master’s degree and PhD from Graduate School of Pharmaceutical Sciences, Osaka University. Meanwhile, he is the Lecturer at Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Thailand. His research interests are Pharmaceutical Quality Control, Antisense Therapy and Vaccine Technology. He has contributed greatly to understand the stability of antibiotics in peritoneal dialysis solutions.
Abstract:
Peritonitis is one of the most serious complication of peritoneal dialysis (PD), causing significant morbidity and occasional mortality. Intraperitoneal (IP) administration of antibiotics is recommended for the treatment of PD-related peritonitis. The beta lactam/beta-lactamase inhibitor such as cefoperazone/sulbactam (sulperazone) has been used in intra-abdominal infections. This combination has a spectrum covering aerobic, facultative and anaerobic bacteria. The purpose of this study was used to determine the stability of cefoperazone (1 g/L) and sulbactam (500 mg/L) in PD solutions (including Extraneal, Dianeal and CAPD/DPCA) by using high performance liquid chromatography (HPLC). The results show that cefoperazone and sulbactam retained more than 90% of their initial concentration for 120 hours when stored at 4°C in whole PD solutions. At room temperatures (25 and 30°C), cefoperazone is reported to be stable in PD solutions at least 24 hours. Nevertheless, sulbactam is reported to be less stable than 24 hours. At body temperature (37°C), cefoperazone was stable in PD fluids less than 24 hours. However, HPLC chromatogram showed sulbactam degradation product and was less stable than 4 hours in icodextrin and glucose PD solutions. Therefore, cefoperazone is stable in PD solutions and can be administered in PD bag for treatment of PD-related peritonitis. However, admixture sulbactam in PD solutions must be used with caution due to its lack of stability. This study provides precious data to healthcare professionals to help make their decisions for preparing and storing these antibiotics under appropriate conditions before administration.







