Day 2 :
- Pharmaceutical Reasearch, Development and Technology | Advances and Applications in HPLC Techniques | Analytical Chemistry | Quality Control, Quality Assurance and Regulatory Filings | Pharmaceutical Nanotechnology | Methods of Chromatography | Drug Formulation & Drug Design Pre-Formulation | Novel Detection Technologies and Drug Discovery
Location: Fleming’s 7+8

Chair
Rucha Majmundar Mehta
R Q Consultants, India
Session Introduction
Gupta P D
Manipal University, India
Title: Chronotherapeutically active formulations of valsartan to treat early morning surge in blood pressure

Biography:
Gupta P D was associated with many prestigious institutions in India (AIIMS, New Delhi, CCMB, Hyderabad) and abroad (Canada, USA, Thailand, England, Japan, France, Czech, Germany, Bulgaria) in various capacities. He is fellow of many Indian and foreign academies and learned societies. He has delivered guest lectures and public lectures in 60 countries. He has established 2 research institutions; on Cataract (Ahmadabad) and Aging Research (Rajkot) in India. Three foreign Post-Doctoral fellows worked along with him in India. He has guided PhD students in seven different subjects including Mechanical Engineering. He has also developed many new techniques and got 4 patents to his credit. He has published more than 250 papers and 22 books and many popular science articles; many of which were translated into Indian and foreign languages. He has received Best Invention and Lifetime Achievement Awards. His life sketch is published in “Marquis Who’s Who” in the world.
Abstract:
In the majority of hypertensive individuals, blood pressure (BP) rises in the early morning hours which lead to serious cardiovascular complications. Currently, available medicines will not meet the blood plasma level of the drug during the morning surge. Chronotherapeutically active formulations rely on unique technologies to deliver a proportion of the daily dose to the time of day when BP rises to peak or near peak levels. The purpose of this study was to develop programmed release dosage forms of valsartan for controlled delivery. The compression coated tablets of valsartan were developed using guar and xanthan gum as coating polymers and studied for physical characteristics, erosion study, in vitro release, ex vivo continuous dissolution absorption and stability study in rabbits. Compression coated tablets with guar gum-lactose (15:85), and xanthan gum: lactose (50:50) showed optimum lag time of 7±0.5 h, 6±0.5 h lag time respectively and shelf lives of the formulations were about 2 years. Absorption of the valsartan from marketed tablet was rapid, whereas absorption was delayed in the developed formulations with clear lag time which was the desired objective of the developed formulation. The optimized formulations showed improved bioavailability and pharmacokinetic profile in rabbits. Thus the present formulation provides therapeutic dosage of valsartan in the morning hours.
Douye P Markmanuel
Isaac Jasper Boro College of Education, Nigeria
Title: Health risk of lead poisoning in four edible snail samples obtained from Bayelsa State, Nigeria

Biography:
Douye P Markmanuel obtained her BEd in Chemistry (2nd class upper) in 2002 from the University of Ibadan, Nigeria, MSc (2011), and PhD (2016) in Environmental Chemistry, University of Port-Harcourt, Nigeria. Her working life is centered on Education and Research. She was employed as Master Grade II Teacher in Bayelsa State college of Arts and Science, (2005) where she taught Chemistry in the Senior Secondary Section. In 2006, she was upgraded to the rank of an Instructor in the main college. Following the establishment of Bayelsa State College of Education, Sagbama, she became an Assistant Lecturer in the college. Presently, she is Lecturer I and Head of Chemistry Department. She is a member, and also holds positions in several professional bodies. She has attended several local and international conferences and workshops. Her current research area is Heavy Metals and Health Risks Hazards in Edible Snail Species.
Abstract:
Lead over the years has been a major environmental nuisance, and lead poisoning is a significant epidemic in many countries in the world including Nigeria. Most often, lead poisoning has been identified as a chronic environmental disease which later develops long-term adverse health effects. However, this study investigated the concentration, fractionation, and potential health risk of lead in four edible snails (A. achatina, L. flammea, P. aurita, and T. fuscatus) obtained from Bayelsa State, Nigeria using Flame Atomic Absorption Spectrometer (FAAS). The mean concentrations of lead (mg/kg dry wet basis, mean±SD) were: A. achatina (29.5±5.41), L. flammea (8.00±1.00), P. aurita (37.7±2.47), and T. fuscatus (27.8±2.89). These values were higher than the permissible limits of FAO/WHO and FEPA. Speciation analysis showed that the water soluble fraction were below the limits of WHO and FEPA. Polar and non-polar fraction were below detection limits (BDL), indicating non-availabilities of polar and non-polar lead species in the snails, while the residual fraction were higher than the acceptable limits of WHO and FEPA. Health risk assessments results revealed that the chronic daily intake (CDI) of lead in the snails were in the decreasing order of P. aurita > A. achatina > T. fuscatus > L. flammea with values of 15.52, 12.14, 11.14, and 3.29 respectively. These values are higher than the provisional daily intakes of lead set by WHO and FEPA. The non-carcinogenic health risks of lead in the snails were generally low (THQ=HI<1), indicating non-cancer adverse health risk at the moment. However, the carcinogenic risk index of lead in the snails was within the threshold values of 1.0x10-6-1.0x10-4 set by USEPA. Therefore, considering the bioacculative nature of lead, these snails should be consumed moderately.
Boyd L. Summers
Weber State University, USA
Title: Ensure Quality Assurance for Companies and Institutions

Biography:
Dr. Boyd L. Summers has completed his Bachelor of Science (BS), Business Administration at Weber State University, USA. Areas of emphasis: Information Systems, Production and Operations Management, Quantitative Analysis and Methods, Human Resources, Economics, Business Management and Statistical Analysis and Computer Science. He is currently working as a Software Technology Consultant for BL Summers Consulting LLC located in Florence, Arizona. With 30 years of experience in Software Engineering and Quality Control and a leader of multiple development teams continues to solve complex technical challenges to ensure that Quality Control problems are addressed, resolved and compliant.
Abstract:
Outside or inside quality assurance representatives are trained and chartered to partner with companies and/or institutions and instill quality, maintain process and product requirement compliance thru in-house audits and evaluations and to provide oversight. Quality is inclusive for creating a community working together and establishes an inspired future for business management, employees and customers. Drive the growth of our people and our business through personal and professional development focused on disciplined execution and quality. At the start of each review period, auditors prepare for audit and evaluation planning by identifying contracts and those processes that will be evaluated during that specific review period.The purpose of the audits and evaluations ensure that activities and/or tasks are completed as planned and are compliant with approved company and/or institution plans and procedures. Companies and/or institutions maintain historical records (electronic or paper) such that they accurately reflect the activities and status they represent. Manage configuration and control of audit and evaluation records as required by company requirements are retained records for compliance and use for future improvements. There are other and effective methods for audits and evaluations, but the number one method is to ensure “Quality Assurance is First” and the other methods come in second!
Dwiyati Pujimulyani
Mercu Buana University, Indonesia
Title: In vitro assay of antioxidant and antidiabetic potency white saffron (curcuma mangga val.) extract and its fractions

Biography:
Dwiyati Pujimulyani has completed her PhD at the age of 46 years and Prof at the age of 49 years from Departement of Food Science, Faculty of Agroindustry, Mercu Buana University, Yogyakarta, Indonesia. She is a lecturer at Mercu Buana University. She has published more than 15 papers in reputed journals.
Abstract:
Diabetes is known as the most common endocrinal disorder indicated by hyperglycemia and long term complications. Oxidative stress and excess of free radicals have been documented in diabetes occurence. Investigation of natural antidiabetic agents and antioxidants with less side effect is therefore needed. Antidiabetic and antihypertensive activities of white saffron (Curcuma mangga) have been reported. In this study (in vitro method), antidiabetic activity of four fractions of C. mangga extract (water, hexane, ethyl acetate, and buthanol fraction) was measured by α/β glucosidase activity assay, while antioxidant activity of those fractions was measured using nitrite oxide (NO) and H2O2-scavenging activity assay. These fractions were also compared to antidiabetic drug, acarbose, as control. Hexane fraction of C. mangga extract showed the highest α-glucosidase inhibitory activity (68.29%; IC50 = 183.21 µg/mL). Ethyl acetate fraction of C. mangga extract showed the highest β-glucosidase inhibitory (81.58%; IC50 = 163.29 µg/mL) and H2O2-scavenging activity (135.69%; IC50 = 265.66 µg/mL). However, acarbose exhibited the highest NO-scavenging activity (74.75%; IC50 = 165.92 µg/mL) compared to all fractions of extract. To conclude, C. mangga extract could be used as alternative in the development of antidiabetic medicine.

Biography:
Abstract:
The manufacture of food products and dietary supplements using natural food colorants has been attracted attention in modern food industry. Carotenoids and anthocyanins as natural colorants show strong antioxidant and immunomodulation activities and may prevent degenerative diseases as well.The present research concerns the development of extraction procedure of carotenoids and anthocyanins containing agro-industrial waste materials (tangerine, orange peel and grape skin). Extractions were carried out in a dynamic supercritical fluid - carbon dioxide (SC-CO2) extraction system. The main carotenoids - beta-carotene, lycopene and anthocyanins obtained in organic extracts were quantified using new, rapid, effective and selective developed and validated HPLC methods. The effects of operating pressure and temperature, extraction time, flow rate of the SC-CO2, sample size and solvent nature used were investigated. The optimal conditions for extraction were found.The two methods for carotenoids and anthocyanins were validated with respect to system suitability test, specificity, linearity-range, accuracy, precision, limit of detection (LOD) and quantitation (LOQ). The stability of solutions were studied as well.The calibration curve is linear over a concentration range 0.08-6.50 µg/mL for beta-carotene (r2=0.9992), 0.34-18.76 µg/mL for lycopene (r2=0.9999); 0.04-40.0 µg/mL and 0.12-40.0 µg/mL for total anthocyanins expressed as cyanidine chloride (r2=0.9999) and kuromanine chloride (r2=0.9999); The LOD and the LOQ are 0.08 µg/mL and 0.04 µg/mL for beta-carotene, 0.34 µg/mL and 0.80 µg/mL for lycopene, 0.04 µg/mL and 0.08 µg/mL for cyaniding chloride; 0.12 µg/mL and 0.16 µg/mL for kuromanine chloride. No interference was observed; The average recovery equals to 106.8 % for beta-carotene, 101.4 % for lycopene, 95.62 % for cyanidine chloride and 94.9 % for kuromanine chloride.The content of each carotenoid per 1 g of dried agro-industrial waste material varies for beta-carotene 0.445 – 3.972 µg (tangerine peel), 0.833 – 2.455 µg (orange peel), for lycopene 0.051 – 179.988 µg (tangerine peel), 0.091 – 0.114 µg (orange peel), for total anthocyanines 4.06 – 56.9 µg (grape skin).
Li Li
The University of Queensland, Australia
Title: Combinational strategy via co-delivery of drugs and sirna by layered double hydroxide-based nanocomposites in cancer therapy

Biography:
Dr Li Li is currently an Advance Queensland Research Fellow (Mid) at Australian Institute for Bioengineering and Nanotechnology. She is a materials scientist with extensive experience in nanoparticle synthesis and applications in targeted drug delivery and vaccination. She has developed several functional NPs platforms including layered double hydroxides (LDHs), silica NPs and nanoemulsions, and applied these NPs to efficiently deliver anti-cancer drugs and siRNA for cancer treatment. She has employed LDH-based nanoparticles to co-deliver drugs and gene to improve drug efficiency in cancer treatment. This new strategy provides a promising approach for advance cancer therapy. She established the close relationships with the national and international experts, published high quality research papers in Adv Mater, Biomaterials, Nano Letters, Nano research, Adv Funct Mater
Abstract:
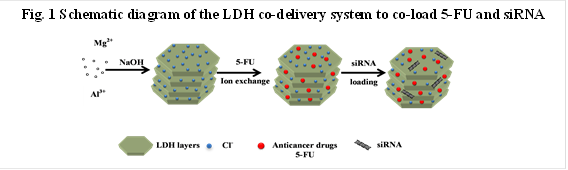
Chemotherapy is one of most common cancer treatments in clinics. In most cases, the clinical responses show that the efficacy of chemotherapy is limited by the development of multidrug resistance (MDR) in cancer cells during a long period of treatment. Target-specific delivery and sustained release of anticancer agents and siRNA has attracted considerable research interest in cancer chemotherapy. It is clear that the single treatment by either anticancer drug or siRNA delivered by nanocarriers can only achieve limited success in overcoming the MDR of cancer cells. Thus, the development of an effective strategy to overcome the multidrug resistance in chemotherapy remains a major challenge in the treatment of cancers, where co-delivery of anticancer drugs and siRNA would be a promising strategy.For this purpose, layered double hydroxides (LDHs), a family of anionic clay materials, have been examined as an example for simultaneous drug and gene delivery by using their unique properties. Our strategy is to combine two different types of anticancer therapeutics for effective cancer treatment. For example, 5-fluorouracil (5-FU) and siRNAs were co-loaded and then co-delivered to treat cancer cells, as illustrated in Scheme 1. Our data clearly indicate that LDH nanoparticles (NPs) can efficiently co-deliver 5-FU and siRNA into MCF-7 and U2OS cells and combination treatment with siRNA and 5-FU leads to significantly higher cytotoxicity to three cancer cell lines (MCF-7, U2OS and HCT-116), compared to the single treatment with either siRNA or 5-FU.Therefore, co-delivery of siRNAs and anticancer drugs by LDHs synergistically enhances the efficacy in these cancer treatments and has great potential as a novel approach for effective cancer treatment.
Biography:
Wael Ebied has completed his BPharm from Tanta University with Postgraduate studies from Al-Azhar University School of Pharmacy. He is a Professional QA, Product Transfer, Tech Support & Clinical in Abbott Laboratories S.A. Middle East, Africa, Pakistan, Turkey and CIS (Commonwealth Independent States) - Established Pharmaceuticals (EPD). He has published many papers in reputed journals and has been serving as an Editorial Board Member of repute. He has more than twenty years’ experience in pharmaceutical industries, biotechnology, clinical trials, medical devices, APIs and herbal medicine. He is an accomplished technical presenter with numerous projects, scientific publications, participated in some patents and was awarded many premiums.
Abstract:
People have tamed animals and domesticated plants for more than 10,000 BC, utilizing specific breeding or simulated selection (as stood out from natural selection). The procedure of selective breeding is the most established type of hereditary adjustment by people, in which organisms with sought traits (and in this way with the desired genes) are utilized to breed the next generation to come keeping in mind living organisms without the coveted characteristic are not reproduced. A genetically modified organism (GMO) is any life form whose genome has been adjusted utilizing genetic engineering procedures. GMOs are utilized as a part of biomedical research, development of pharmaceuticals, and testing gene therapy. The expression GMO does not generally infer, but rather can incorporate, focused on insertions of genes from one species into another. Genetically modified animals currently being developed can be used for research concerning human diseases so as to develop a model animal with the desired diseases to be studied. Transgenic animals are used as experimental models to demonstrate phenotypic aspects and for testing drugs in biomedical research. Genetically engineered animals are turning out to be more crucial to the revelation and improvement of cures and medicines for some genuine illnesses.Zoopharmacognosy is a behavior in which animals perform self-medication through selecting and ingesting or topically applying plants, soils, insects, and psychoactive drugs to treat or prevent illnesses. Animals ingest non-consumable materials such as clay, charcoal and even toxic plants, apparently to prevent parasitic infestation or poisoning. Self-medication in wild animals remains a controversial subject because evidence is in most cases circumstantial or anecdotal, however, there are many reported examples. The techniques by which living organisms self-sedate fluctuate, however can be arranged by as prophylactic (protection. before contamination or harming) or therapeutic (after disease, to battle the pathogen or harming). In spite of the fact that the basic mental and physiological mechanisms of such learned self-medicating behavior are vague, its versatile esteem is proposed to be far reaching, incorporating diseased-laboratory animals. In light of the development of parasites and pathogens resistance to manufactured medications, the investigation of animal self-medication and ethno-medicine offers a novel line of examination to give ecologically-sound strategies to the treatment of diseases utilizing plant-based meds.The objective of this research article is: How human diseased-animal models will keep themselves well in an artificial wild and what we can learn from their self-medication approaches in screening new therapeutics for human diseases. The current proposal is to test the hypothesis that zoopharmacognosy is operational with model organisms in artificial wild life. Once a molecular target of disease is revealed, one can use this perspective for identifying active ingredient(s) from natural medicine in new drug discovery. The generation of transgenic animals by biotechnological techniques will provide human disease models for screening drugs of clinical interest with the help of zoo pharmacognosy. Some of the compounds have been identified by zoopharmacognosy were found to kill parasitic worms, and some other chemicals may be useful in fighting tumor cells growth. There is no question that the templates for most drugs are in the natural world. The question is how to discover using zoopharmacognosy by human diseased-animal models.







