Day 2 :
- Pharmaceutical Reasearch, Development and Technology | Advances and Applications in HPLC Techniques | Analytical Chemistry | Quality Control, Quality Assurance and Regulatory Filings | Pharmaceutical Nanotechnology | Methods of Chromatography
Location: Fleming’s 7+8

Chair
Salah M Blaih
Kent State University, USA

Co-Chair
M Sharaf El-Din
University of Mansoura, Egypt
Session Introduction
Salah M Blaih
Kent State University, USA
Title: Personalized cancer therapeutics: Updates on drug developments and characterization of biosimilars

Biography:
Salah M Blaih has been involved in teaching, research and authoring in pharmacy and chemistry for more than 25 years. He was elected to the USP’s Council of Expert’s Committee on Gastrointestinal, Renal, and Endocrine and is a world-wide invited speaker on quality of medicines/compendia standards, pharmacogenomics, and medication safety. He is a Fellow of The Royal Society of Chemistry, a two-time president of The American Chemical Society-Penn Ohio Section, and a board-certified pharmacist with biographical entries in Who’s Who in the World and Who’s Who in America.
Abstract:
Cancer biomarkers are relevant for identifying patients who are likely to benefit from a given treatment (right drug for the right patient). This approach is utilized in cancer drug development as well as in measuring patient’s response to therapy. Targeted therapies are designed to interfere with molecular targets with a goal of more precision and fewer side effects. These molecular pathways are broadly classified as either 1) monoclonal antibodies that target transmembrane receptors or extracellular growth factors or 2) small molecules that penetrate cell membrane and block and interfere with the enzymatic activity of target proteins. Emerging cancer therapeutics, which act on specific molecular targets, their mutations, and their role in tumor progression are presented: 1) PI3K, CDK, and PARP inhibitors and 2) PD-1 (programmed death receptor binding). Some aspects of development, characterization, and regulation of biosimilars as implemented by the FDA and EMEA are also presented.
M Sharaf El-Din
University of Mansoura, Egypt
Title: Spectrophotometric methods for simultaneous determination of rivaroxaban and clopidogrel in their binary mixture

Biography:
Professor Dr.Mohie Sharaf El Din has completed his PhD at the age of 32 years from Bonn -University – Germany 1982 and post-doctoral studies from Graz Institute of pharmaceutical chemistry and biochemistry 1986 - Austria. He was the Dean of Faculty of Pharmacy – Heliopolis University – Cairo-Egypt 2015, Head of Analytical chemistry Department, Faculty of Pharmacy, and Mansoura University – Mansoura – Egypt 1997 -2004. He has published more than 100 papers in reputed journals and has been serving as an editorial board member of repute.( as an editorial board member of Mansoura Journal of pharmaceutical Science , Faculty of Pharmacy - Mansoura – Egypt, as the chair/co-chair for the session of many conferences and workshops )
Abstract:
Three rapid, accurate and very simple derivative spectrophotometric methods for RIV and CLP assay in their binary mixture and tablet dosage forms were developed. Method (I) is first derivative spectrophotometric method, derivative amplitudes were measured at the zero crossing wavelength of 289 and 249.5 nm for determination of RIV and CLP, respectively. The calibration curve is rectilinear over the range 2.0-20.0 µg/ml for RIV and 5.0-60.0 µg/ml for CLP with LOD of 0.211 and 0.361 μg mL-1 and LOQ of 0.641 and 1.095 μg mL-1 for RIV and CLP, respectively. Method (II) is ratio derivative spectrophotometric method. The ratio spectra of each drug were derived by dividing its spectra on a constant concentration of the other drug as a divisor. Derivative amplitudes were measured at 256 nm for RIV and at 214.5 nm for CLP over the same concentration range as the first method with LOD of 0.137 and 0.485 μg mL-1 and LOQ of 0.417and 1.471 μg mL-1 for RIV and CLP, respectively. Method (III) is absorbance ratio method, absorbance of both drugs were recorded at two wavelengths λ1 (232) iso-absorptive point and λ2 (249) λmax of RIV. The final concentrations were obtained by applying the Q equations. The method was linear over the same concentration range as the first method with LOD of 0.272 and 0.485 μg mL-1 and LOQ of 0.826 and 1.471 μg mL-1 for RIV and CLP, respectively. The proposed methods were validated as per International Conference of Harmonization guidelines. The proposed methods were successfully applied to both drugs analysis in their laboratory prepared co-formulated tablet. Statistical comparison of the results with those of the reference method illustrate good agreement and confirm that there were no significant difference in the accuracy and precision between the proposed and reference one respectively.
Thomas D Benen
Microtrac GmbH, Germany
Title: Integrating 180° DLS into on-line pharmaceutical processes and high-throughput robotics

Biography:
Thomas D Benen has completed his PhD at the University of Hamburg and Postoctoral Research at the University of Regensburg, investigating the formation of viral particles. He has worked for some of the major players of the particle characteriztation industry like Malvern Instruments and NanoSight Ltd. At Microtrac GmbH, he is responsible for the business in the D-A-CH region. He is delegate of the ISO group TC24 committed on the develeopment of international standards for particle measurement.
Abstract:
Dynamic Light Scattering (DLS) is a prevalent tool for determining particle size distributions in fine particulate material suspensions, micro-emulsions and nano-scale matter like proteins and drug delivery particles. Usually optical arrangements in lower angles are used that demand heavy dilution of samples. Backscattering, however, allows for considerably higher concentrations and is the right choice for concentrated samples which incur in processes. Furthermore, the 180° backscattering is especially suitable for direct in-line use in reactors, because the handling of the measurement probe head with a diameter of 8 mm is just as easy as a pH head. A probe cap effectively shields the Brownian motion from the process fluidics. At higher concentrations, where particle-particle interactions are present, on-line systems with automated dilution are in use. The external control of our DLS measurement software moreover allows for using robots in High-Throughput Screening (HTS) and fully automated formulation stations. Those work stations, equipped with liquid handling and parallel reactors, perform programmed formulation steps like mixing, stirring, heating and shaking. Apart from the aforementioned particle size distribution, rheological attributes and spectroscopic measurements are also included. Thus DLS has finally arrived as an automatized tool for pharmaceutical processes and HTS.
Roger M Leblanc
University of Miami, USA
Title: Analysis of microbiomolecule-nanoparticle drug delivery system using circular dichroism spectroscopy

Biography:
Roger M. Leblanc received a B.Sc. degree in Chemistry from Université Laval in 1964, followed by a Ph.D. in Physical Chemistry in 1968. Then, he obtained a postdoc position at the Royal Institution of Great Britain for two years before moving to the University of Québec, Trois-Rivières, Canada, where he spent 20+ years of studying photobiophysics. He moved his research to the University of Miami in 1994. Dr. Leblanc is Professor and Chair of Chemistry Department at University of Miami. And his research interests are centered on biophotophysics, spectroscopy and surface chemistry and he has published more than 500 research articles related to these topics and has guided more than 100 Ph.D. and M.Sc.
Abstract:
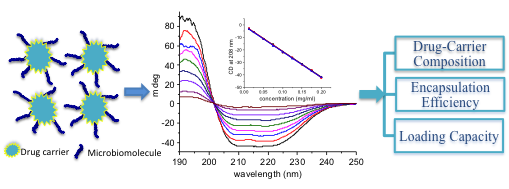
Microbiomolecules such as peptides, proteins and nucleic acids (i.e., DNA and RNA) have become very promising drug candidates in recent decades due to their unique properties and interactions with biological systems. Compared to the traditional small organic molecules (i.e., less than 500 g/mol), these candidates are highly selective due to their rich structure features and unique interaction with their target. However, due to their high enzymatic susceptibility, low membrane permeability and poor bioavailability, applications of these drugs have been significantly limited. Targeted drug delivery by drug delivery systems (DDS) has been the main strategy to overcome the problems (i.e., high enzymatic susceptibility and poor bioavailability) associated with these microbiomolecules drug candidates. In this regard, considerable efforts have been made to design and develop drug delivery systems that could transport these microbiomolecules to site of interest. One important aspect to consider when designing a DDS is the drug-to-carrier ratio since the use of high quantity of carrier could potentially cause a series of problems, such as carrier-related toxicity and the possibility of immune reactions against the carrier in the patient body. However, the ability to determine the composition of a DDS after loading a drug to the carrier is significantly limited by current analytical methods. In this talk, a simple yet convenient method based on circular dichroism (CD) spectroscopy to determine the compositions of the various protein-carrier conjugates will be introduced. Specifically, five important proteins, α1-antitrypsin, hemoglobin human, human serum albumin, human transferrin and r-globulin were chemically conjugated to two model drug carriers, namely carbon dots and polymer O-(2-carboxyethyl) polyethylene glycol; and their compositions were determined with CD spectroscopy. It will also be demonstrated that the composition of nucleic acid conjugates could also be determined using the same methodology.
Nahed El-Najjar
University Hospital Regensburg, Germany
Title: Liquid chromatography-tandem mass spectrometry increases the clinical outcome from the antimicrobial therapy used in intensive care units

Biography:
Abstract:
In the last years, liquid chromatography-tandem mass spectrometry (LC-MS/MS) has become the method of choice for therapeutic drug monitoring (TDM) of drugs used in critically ill patients. This is largely due to the high accuracy and fast delivery of the results obtained with LC-MS/MS which enables quick decision in dose adjustment increasing thus the clinical outcomes of treatments used in Intensive Care Units (ICU) patients. This is particularly of interest when it comes to the antibiotic therapy which is increasingly acknowledged to be frequently unsatisfactory. Inadequacy of the antibiotic therapy is due to the wide range of pathophysiological changes observed in critically ill patients, which impact the proper pharmacokinetic/pharmacodynamic profile of the antibiotics used. Even though the observed poor clinical outcome is mostly related to poor antibiotics exposure, which also increases the risk of acquiring bacterial resistance, dysfunction in kidney and liver, which are the major routes for drug elimination, result in an increased risk of the prompt onset of toxic drug concentration. Therefore, establishing reliable methods for TDM is of utmost importance. When it comes to be applied in routine analysis, analytical methods used for TDM should encompass the simultaneous analysis of different antibiotics widely used in ICU. Challenges encountered during method development as well as the successful application of LC-MS/MS methods for TDM of antibiotics in critically ill patients will be presented and discussed.
Yuan-Po Lee
Chia-Nan University of Pharmacy and Science, Taiwan
Title: The evaluation of stir-bar sorptive extraction and dispersive liquid-liquid microextraction methods for the determination of 20 pharmaceuticals in environmental waste water
Biography:
The Authors are all teaching in Chia-Nan University of Pharmacy and Science and worked on the analytical methods development and validation for more than 20 years. The scope for the analytical methods have aplplied to pharmaceutical, cosmetic and environmental samples and offer industrial services
Abstract:
Stir bar sorptive extraction, SBSE and Dispersive liquid-liquid microextraction, DLLME have attracted much interest due to their simplicity, rapidity of operation by allowing the direct extraction of solutes from sample or low consumption of solvents and reagents during sample pretreament. In this research, SBSE and DLLME were used for the sample pretreament of environmental water samples and followed by gas chromatography/mass spectroscopy analysis for the validation of determining 20 pharmaceuticals in environmental water samples. Fluoxetine, chlorpheniramine, sertraline, methamphetamine, diphenhydramine, amitriptyline, lidocaine, venlafaxine, citalopram, chlorpromazine, verapamil, propoxyphene, promethazine, diazepam, meperidine, methadone, doxylamine, mirtazapine, dextomethorphan and codeine were selected as the target compounds. The results show that most substances can be effectively extracted by SBSE at pH 9.2 except methamphetamine, lidocaine and codeine and by DLLME using toluene as extraction solvent except methamphetamine, propoxyphene, codeine and diazepam. The applicability of the sample pretreatment methods strongly depended on the characteristics of target compounds. Codeine shows very poor recoveries which means it is not suitable to determine its content under selected analytical conditions. It is recommended to use SBSE methods for sample pretreatment because it can achieve lower detection limit, repeated use of stir bar and no need to use extraction solvent. For real environmental water samples, SBSE was used to determine the presence of target compounds and evaluate the matrix effect of the real samples. The results show that only lidocaine was detected in one of the hospital waste samples and methamphetamine, lidocaine and codeine all showed poor recoveries as in standard solution.
Somjing Roongjang
Chiang Mai University, Thailand
Title: The stability of cefoperazone/sulbactam (sulperazone) in PD solutions

Biography:
Somjing Roongjang has completed his Master’s degree and PhD from Graduate School of Pharmaceutical Sciences, Osaka University. Meanwhile, he is the Lecturer at Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Thailand. His research interests are Pharmaceutical Quality Control, Antisense Therapy and Vaccine Technology. He has contributed greatly to understand the stability of antibiotics in peritoneal dialysis solutions.
Abstract:
Peritonitis is one of the most serious complication of peritoneal dialysis (PD), causing significant morbidity and occasional mortality. Intraperitoneal (IP) administration of antibiotics is recommended for the treatment of PD-related peritonitis. The beta lactam/beta-lactamase inhibitor such as cefoperazone/sulbactam (sulperazone) has been used in intra-abdominal infections. This combination has a spectrum covering aerobic, facultative and anaerobic bacteria. The purpose of this study was used to determine the stability of cefoperazone (1 g/L) and sulbactam (500 mg/L) in PD solutions (including Extraneal, Dianeal and CAPD/DPCA) by using high performance liquid chromatography (HPLC). The results show that cefoperazone and sulbactam retained more than 90% of their initial concentration for 120 hours when stored at 4°C in whole PD solutions. At room temperatures (25 and 30°C), cefoperazone is reported to be stable in PD solutions at least 24 hours. Nevertheless, sulbactam is reported to be less stable than 24 hours. At body temperature (37°C), cefoperazone was stable in PD fluids less than 24 hours. However, HPLC chromatogram showed sulbactam degradation product and was less stable than 4 hours in icodextrin and glucose PD solutions. Therefore, cefoperazone is stable in PD solutions and can be administered in PD bag for treatment of PD-related peritonitis. However, admixture sulbactam in PD solutions must be used with caution due to its lack of stability. This study provides precious data to healthcare professionals to help make their decisions for preparing and storing these antibiotics under appropriate conditions before administration.
- Pharmaceutical Reasearch, Development and Technology | Advances and Applications in HPLC Techniques | Analytical Chemistry | Quality Control, Quality Assurance and Regulatory Filings | Pharmaceutical Nanotechnology | Methods of Chromatography | Drug Formulation & Drug Design Pre-Formulation | Novel Detection Technologies and Drug Discovery
Location: Fleming’s 7+8

Chair
Rucha Majmundar Mehta
R Q Consultants, India
Session Introduction
Gupta P D
Manipal University, India
Title: Chronotherapeutically active formulations of valsartan to treat early morning surge in blood pressure

Biography:
Gupta P D was associated with many prestigious institutions in India (AIIMS, New Delhi, CCMB, Hyderabad) and abroad (Canada, USA, Thailand, England, Japan, France, Czech, Germany, Bulgaria) in various capacities. He is fellow of many Indian and foreign academies and learned societies. He has delivered guest lectures and public lectures in 60 countries. He has established 2 research institutions; on Cataract (Ahmadabad) and Aging Research (Rajkot) in India. Three foreign Post-Doctoral fellows worked along with him in India. He has guided PhD students in seven different subjects including Mechanical Engineering. He has also developed many new techniques and got 4 patents to his credit. He has published more than 250 papers and 22 books and many popular science articles; many of which were translated into Indian and foreign languages. He has received Best Invention and Lifetime Achievement Awards. His life sketch is published in “Marquis Who’s Who” in the world.
Abstract:
In the majority of hypertensive individuals, blood pressure (BP) rises in the early morning hours which lead to serious cardiovascular complications. Currently, available medicines will not meet the blood plasma level of the drug during the morning surge. Chronotherapeutically active formulations rely on unique technologies to deliver a proportion of the daily dose to the time of day when BP rises to peak or near peak levels. The purpose of this study was to develop programmed release dosage forms of valsartan for controlled delivery. The compression coated tablets of valsartan were developed using guar and xanthan gum as coating polymers and studied for physical characteristics, erosion study, in vitro release, ex vivo continuous dissolution absorption and stability study in rabbits. Compression coated tablets with guar gum-lactose (15:85), and xanthan gum: lactose (50:50) showed optimum lag time of 7±0.5 h, 6±0.5 h lag time respectively and shelf lives of the formulations were about 2 years. Absorption of the valsartan from marketed tablet was rapid, whereas absorption was delayed in the developed formulations with clear lag time which was the desired objective of the developed formulation. The optimized formulations showed improved bioavailability and pharmacokinetic profile in rabbits. Thus the present formulation provides therapeutic dosage of valsartan in the morning hours.
Douye P Markmanuel
Isaac Jasper Boro College of Education, Nigeria
Title: Health risk of lead poisoning in four edible snail samples obtained from Bayelsa State, Nigeria

Biography:
Douye P Markmanuel obtained her BEd in Chemistry (2nd class upper) in 2002 from the University of Ibadan, Nigeria, MSc (2011), and PhD (2016) in Environmental Chemistry, University of Port-Harcourt, Nigeria. Her working life is centered on Education and Research. She was employed as Master Grade II Teacher in Bayelsa State college of Arts and Science, (2005) where she taught Chemistry in the Senior Secondary Section. In 2006, she was upgraded to the rank of an Instructor in the main college. Following the establishment of Bayelsa State College of Education, Sagbama, she became an Assistant Lecturer in the college. Presently, she is Lecturer I and Head of Chemistry Department. She is a member, and also holds positions in several professional bodies. She has attended several local and international conferences and workshops. Her current research area is Heavy Metals and Health Risks Hazards in Edible Snail Species.
Abstract:
Lead over the years has been a major environmental nuisance, and lead poisoning is a significant epidemic in many countries in the world including Nigeria. Most often, lead poisoning has been identified as a chronic environmental disease which later develops long-term adverse health effects. However, this study investigated the concentration, fractionation, and potential health risk of lead in four edible snails (A. achatina, L. flammea, P. aurita, and T. fuscatus) obtained from Bayelsa State, Nigeria using Flame Atomic Absorption Spectrometer (FAAS). The mean concentrations of lead (mg/kg dry wet basis, mean±SD) were: A. achatina (29.5±5.41), L. flammea (8.00±1.00), P. aurita (37.7±2.47), and T. fuscatus (27.8±2.89). These values were higher than the permissible limits of FAO/WHO and FEPA. Speciation analysis showed that the water soluble fraction were below the limits of WHO and FEPA. Polar and non-polar fraction were below detection limits (BDL), indicating non-availabilities of polar and non-polar lead species in the snails, while the residual fraction were higher than the acceptable limits of WHO and FEPA. Health risk assessments results revealed that the chronic daily intake (CDI) of lead in the snails were in the decreasing order of P. aurita > A. achatina > T. fuscatus > L. flammea with values of 15.52, 12.14, 11.14, and 3.29 respectively. These values are higher than the provisional daily intakes of lead set by WHO and FEPA. The non-carcinogenic health risks of lead in the snails were generally low (THQ=HI<1), indicating non-cancer adverse health risk at the moment. However, the carcinogenic risk index of lead in the snails was within the threshold values of 1.0x10-6-1.0x10-4 set by USEPA. Therefore, considering the bioacculative nature of lead, these snails should be consumed moderately.
Boyd L. Summers
Weber State University, USA
Title: Ensure Quality Assurance for Companies and Institutions

Biography:
Dr. Boyd L. Summers has completed his Bachelor of Science (BS), Business Administration at Weber State University, USA. Areas of emphasis: Information Systems, Production and Operations Management, Quantitative Analysis and Methods, Human Resources, Economics, Business Management and Statistical Analysis and Computer Science. He is currently working as a Software Technology Consultant for BL Summers Consulting LLC located in Florence, Arizona. With 30 years of experience in Software Engineering and Quality Control and a leader of multiple development teams continues to solve complex technical challenges to ensure that Quality Control problems are addressed, resolved and compliant.
Abstract:
Outside or inside quality assurance representatives are trained and chartered to partner with companies and/or institutions and instill quality, maintain process and product requirement compliance thru in-house audits and evaluations and to provide oversight. Quality is inclusive for creating a community working together and establishes an inspired future for business management, employees and customers. Drive the growth of our people and our business through personal and professional development focused on disciplined execution and quality. At the start of each review period, auditors prepare for audit and evaluation planning by identifying contracts and those processes that will be evaluated during that specific review period.The purpose of the audits and evaluations ensure that activities and/or tasks are completed as planned and are compliant with approved company and/or institution plans and procedures. Companies and/or institutions maintain historical records (electronic or paper) such that they accurately reflect the activities and status they represent. Manage configuration and control of audit and evaluation records as required by company requirements are retained records for compliance and use for future improvements. There are other and effective methods for audits and evaluations, but the number one method is to ensure “Quality Assurance is First” and the other methods come in second!
Dwiyati Pujimulyani
Mercu Buana University, Indonesia
Title: In vitro assay of antioxidant and antidiabetic potency white saffron (curcuma mangga val.) extract and its fractions

Biography:
Dwiyati Pujimulyani has completed her PhD at the age of 46 years and Prof at the age of 49 years from Departement of Food Science, Faculty of Agroindustry, Mercu Buana University, Yogyakarta, Indonesia. She is a lecturer at Mercu Buana University. She has published more than 15 papers in reputed journals.
Abstract:
Diabetes is known as the most common endocrinal disorder indicated by hyperglycemia and long term complications. Oxidative stress and excess of free radicals have been documented in diabetes occurence. Investigation of natural antidiabetic agents and antioxidants with less side effect is therefore needed. Antidiabetic and antihypertensive activities of white saffron (Curcuma mangga) have been reported. In this study (in vitro method), antidiabetic activity of four fractions of C. mangga extract (water, hexane, ethyl acetate, and buthanol fraction) was measured by α/β glucosidase activity assay, while antioxidant activity of those fractions was measured using nitrite oxide (NO) and H2O2-scavenging activity assay. These fractions were also compared to antidiabetic drug, acarbose, as control. Hexane fraction of C. mangga extract showed the highest α-glucosidase inhibitory activity (68.29%; IC50 = 183.21 µg/mL). Ethyl acetate fraction of C. mangga extract showed the highest β-glucosidase inhibitory (81.58%; IC50 = 163.29 µg/mL) and H2O2-scavenging activity (135.69%; IC50 = 265.66 µg/mL). However, acarbose exhibited the highest NO-scavenging activity (74.75%; IC50 = 165.92 µg/mL) compared to all fractions of extract. To conclude, C. mangga extract could be used as alternative in the development of antidiabetic medicine.

Biography:
Abstract:
The manufacture of food products and dietary supplements using natural food colorants has been attracted attention in modern food industry. Carotenoids and anthocyanins as natural colorants show strong antioxidant and immunomodulation activities and may prevent degenerative diseases as well.The present research concerns the development of extraction procedure of carotenoids and anthocyanins containing agro-industrial waste materials (tangerine, orange peel and grape skin). Extractions were carried out in a dynamic supercritical fluid - carbon dioxide (SC-CO2) extraction system. The main carotenoids - beta-carotene, lycopene and anthocyanins obtained in organic extracts were quantified using new, rapid, effective and selective developed and validated HPLC methods. The effects of operating pressure and temperature, extraction time, flow rate of the SC-CO2, sample size and solvent nature used were investigated. The optimal conditions for extraction were found.The two methods for carotenoids and anthocyanins were validated with respect to system suitability test, specificity, linearity-range, accuracy, precision, limit of detection (LOD) and quantitation (LOQ). The stability of solutions were studied as well.The calibration curve is linear over a concentration range 0.08-6.50 µg/mL for beta-carotene (r2=0.9992), 0.34-18.76 µg/mL for lycopene (r2=0.9999); 0.04-40.0 µg/mL and 0.12-40.0 µg/mL for total anthocyanins expressed as cyanidine chloride (r2=0.9999) and kuromanine chloride (r2=0.9999); The LOD and the LOQ are 0.08 µg/mL and 0.04 µg/mL for beta-carotene, 0.34 µg/mL and 0.80 µg/mL for lycopene, 0.04 µg/mL and 0.08 µg/mL for cyaniding chloride; 0.12 µg/mL and 0.16 µg/mL for kuromanine chloride. No interference was observed; The average recovery equals to 106.8 % for beta-carotene, 101.4 % for lycopene, 95.62 % for cyanidine chloride and 94.9 % for kuromanine chloride.The content of each carotenoid per 1 g of dried agro-industrial waste material varies for beta-carotene 0.445 – 3.972 µg (tangerine peel), 0.833 – 2.455 µg (orange peel), for lycopene 0.051 – 179.988 µg (tangerine peel), 0.091 – 0.114 µg (orange peel), for total anthocyanines 4.06 – 56.9 µg (grape skin).
Li Li
The University of Queensland, Australia
Title: Combinational strategy via co-delivery of drugs and sirna by layered double hydroxide-based nanocomposites in cancer therapy

Biography:
Dr Li Li is currently an Advance Queensland Research Fellow (Mid) at Australian Institute for Bioengineering and Nanotechnology. She is a materials scientist with extensive experience in nanoparticle synthesis and applications in targeted drug delivery and vaccination. She has developed several functional NPs platforms including layered double hydroxides (LDHs), silica NPs and nanoemulsions, and applied these NPs to efficiently deliver anti-cancer drugs and siRNA for cancer treatment. She has employed LDH-based nanoparticles to co-deliver drugs and gene to improve drug efficiency in cancer treatment. This new strategy provides a promising approach for advance cancer therapy. She established the close relationships with the national and international experts, published high quality research papers in Adv Mater, Biomaterials, Nano Letters, Nano research, Adv Funct Mater
Abstract:
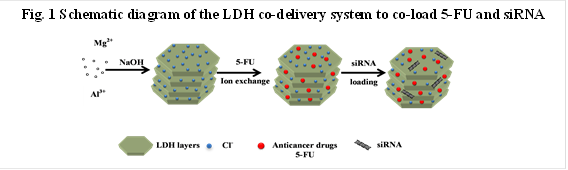
Chemotherapy is one of most common cancer treatments in clinics. In most cases, the clinical responses show that the efficacy of chemotherapy is limited by the development of multidrug resistance (MDR) in cancer cells during a long period of treatment. Target-specific delivery and sustained release of anticancer agents and siRNA has attracted considerable research interest in cancer chemotherapy. It is clear that the single treatment by either anticancer drug or siRNA delivered by nanocarriers can only achieve limited success in overcoming the MDR of cancer cells. Thus, the development of an effective strategy to overcome the multidrug resistance in chemotherapy remains a major challenge in the treatment of cancers, where co-delivery of anticancer drugs and siRNA would be a promising strategy.For this purpose, layered double hydroxides (LDHs), a family of anionic clay materials, have been examined as an example for simultaneous drug and gene delivery by using their unique properties. Our strategy is to combine two different types of anticancer therapeutics for effective cancer treatment. For example, 5-fluorouracil (5-FU) and siRNAs were co-loaded and then co-delivered to treat cancer cells, as illustrated in Scheme 1. Our data clearly indicate that LDH nanoparticles (NPs) can efficiently co-deliver 5-FU and siRNA into MCF-7 and U2OS cells and combination treatment with siRNA and 5-FU leads to significantly higher cytotoxicity to three cancer cell lines (MCF-7, U2OS and HCT-116), compared to the single treatment with either siRNA or 5-FU.Therefore, co-delivery of siRNAs and anticancer drugs by LDHs synergistically enhances the efficacy in these cancer treatments and has great potential as a novel approach for effective cancer treatment.
Biography:
Wael Ebied has completed his BPharm from Tanta University with Postgraduate studies from Al-Azhar University School of Pharmacy. He is a Professional QA, Product Transfer, Tech Support & Clinical in Abbott Laboratories S.A. Middle East, Africa, Pakistan, Turkey and CIS (Commonwealth Independent States) - Established Pharmaceuticals (EPD). He has published many papers in reputed journals and has been serving as an Editorial Board Member of repute. He has more than twenty years’ experience in pharmaceutical industries, biotechnology, clinical trials, medical devices, APIs and herbal medicine. He is an accomplished technical presenter with numerous projects, scientific publications, participated in some patents and was awarded many premiums.
Abstract:
People have tamed animals and domesticated plants for more than 10,000 BC, utilizing specific breeding or simulated selection (as stood out from natural selection). The procedure of selective breeding is the most established type of hereditary adjustment by people, in which organisms with sought traits (and in this way with the desired genes) are utilized to breed the next generation to come keeping in mind living organisms without the coveted characteristic are not reproduced. A genetically modified organism (GMO) is any life form whose genome has been adjusted utilizing genetic engineering procedures. GMOs are utilized as a part of biomedical research, development of pharmaceuticals, and testing gene therapy. The expression GMO does not generally infer, but rather can incorporate, focused on insertions of genes from one species into another. Genetically modified animals currently being developed can be used for research concerning human diseases so as to develop a model animal with the desired diseases to be studied. Transgenic animals are used as experimental models to demonstrate phenotypic aspects and for testing drugs in biomedical research. Genetically engineered animals are turning out to be more crucial to the revelation and improvement of cures and medicines for some genuine illnesses.Zoopharmacognosy is a behavior in which animals perform self-medication through selecting and ingesting or topically applying plants, soils, insects, and psychoactive drugs to treat or prevent illnesses. Animals ingest non-consumable materials such as clay, charcoal and even toxic plants, apparently to prevent parasitic infestation or poisoning. Self-medication in wild animals remains a controversial subject because evidence is in most cases circumstantial or anecdotal, however, there are many reported examples. The techniques by which living organisms self-sedate fluctuate, however can be arranged by as prophylactic (protection. before contamination or harming) or therapeutic (after disease, to battle the pathogen or harming). In spite of the fact that the basic mental and physiological mechanisms of such learned self-medicating behavior are vague, its versatile esteem is proposed to be far reaching, incorporating diseased-laboratory animals. In light of the development of parasites and pathogens resistance to manufactured medications, the investigation of animal self-medication and ethno-medicine offers a novel line of examination to give ecologically-sound strategies to the treatment of diseases utilizing plant-based meds.The objective of this research article is: How human diseased-animal models will keep themselves well in an artificial wild and what we can learn from their self-medication approaches in screening new therapeutics for human diseases. The current proposal is to test the hypothesis that zoopharmacognosy is operational with model organisms in artificial wild life. Once a molecular target of disease is revealed, one can use this perspective for identifying active ingredient(s) from natural medicine in new drug discovery. The generation of transgenic animals by biotechnological techniques will provide human disease models for screening drugs of clinical interest with the help of zoo pharmacognosy. Some of the compounds have been identified by zoopharmacognosy were found to kill parasitic worms, and some other chemicals may be useful in fighting tumor cells growth. There is no question that the templates for most drugs are in the natural world. The question is how to discover using zoopharmacognosy by human diseased-animal models.














